| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
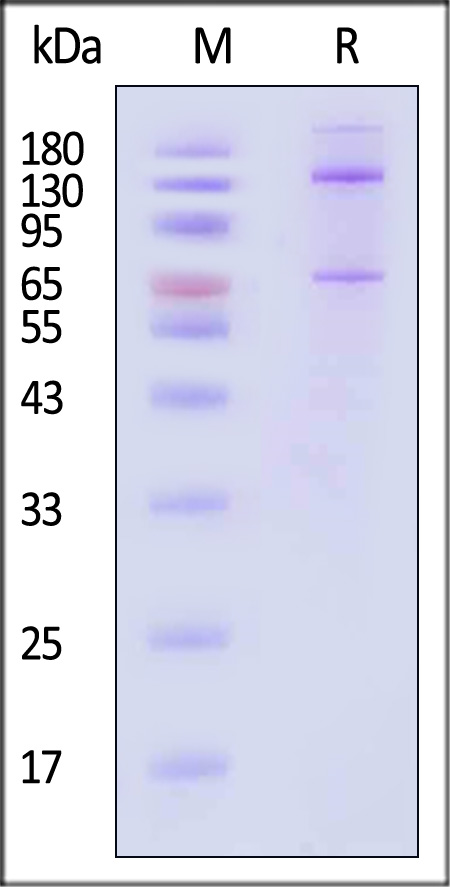
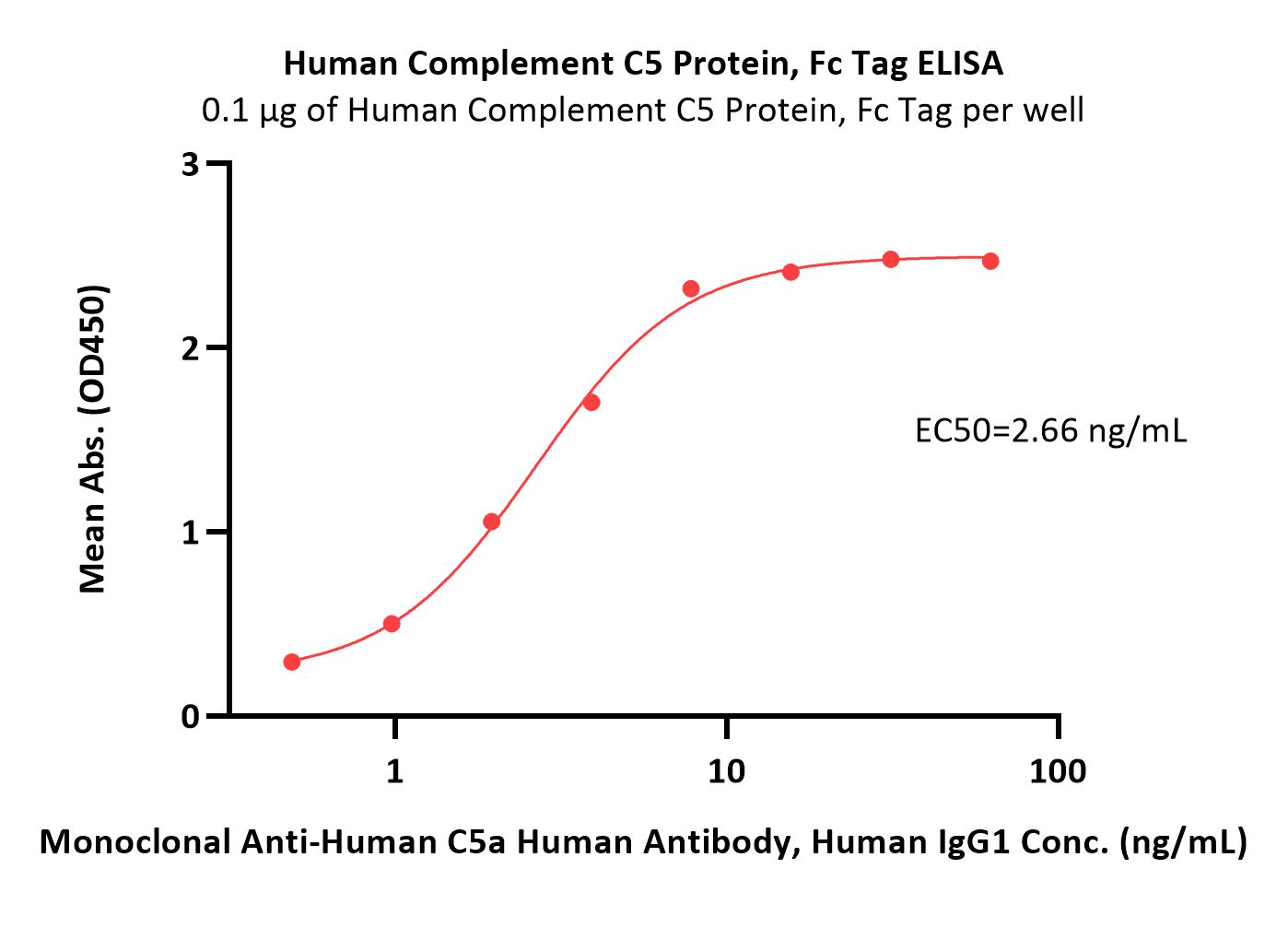
| CO5-H5253 | Human | Human Complement C5 Protein, Fc Tag |  |
|
|
| CO5-H82E9 | Human | Biotinylated Human Complement C5 Protein, His,Avitag™ |  |

|

|
| CO5-R52H4 | Rabbit | Rabbit Complement C5 Protein, His Tag |  |

|
|
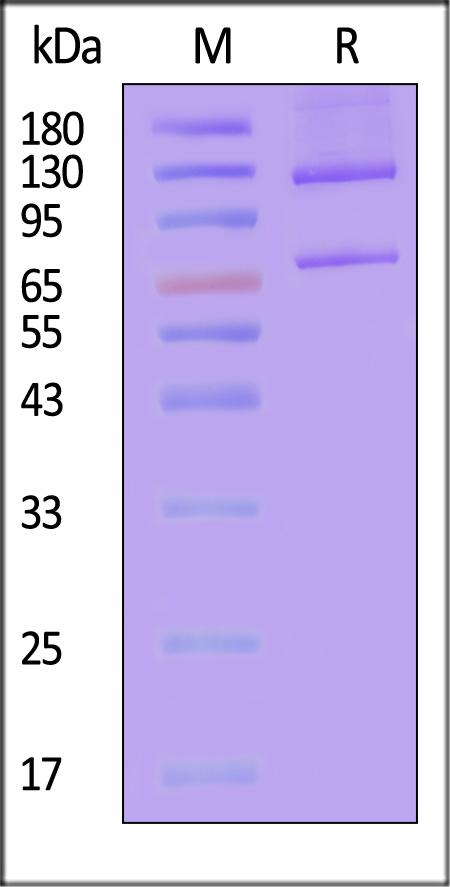
| CO5-H52H7 | Human | Human Complement C5 (w917s) Protein, His Tag |  |
|

|
| CO5-H52Hx | Human | Human Complement C5 (R885H) Protein, His Tag |  |

|

|
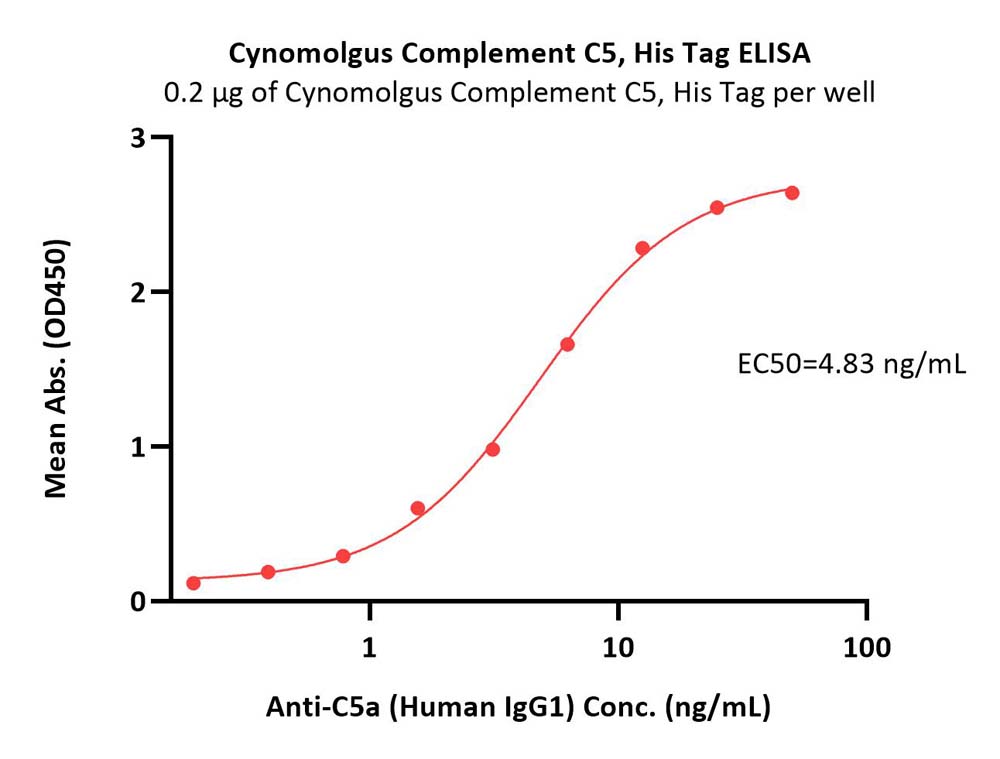
| CO5-C52Hx | Cynomolgus | Cynomolgus Complement C5 Protein, His Tag |  |

|
|
| CO5-R52H5 | Rat | Rat Complement C5 Protein, His Tag |  |

|
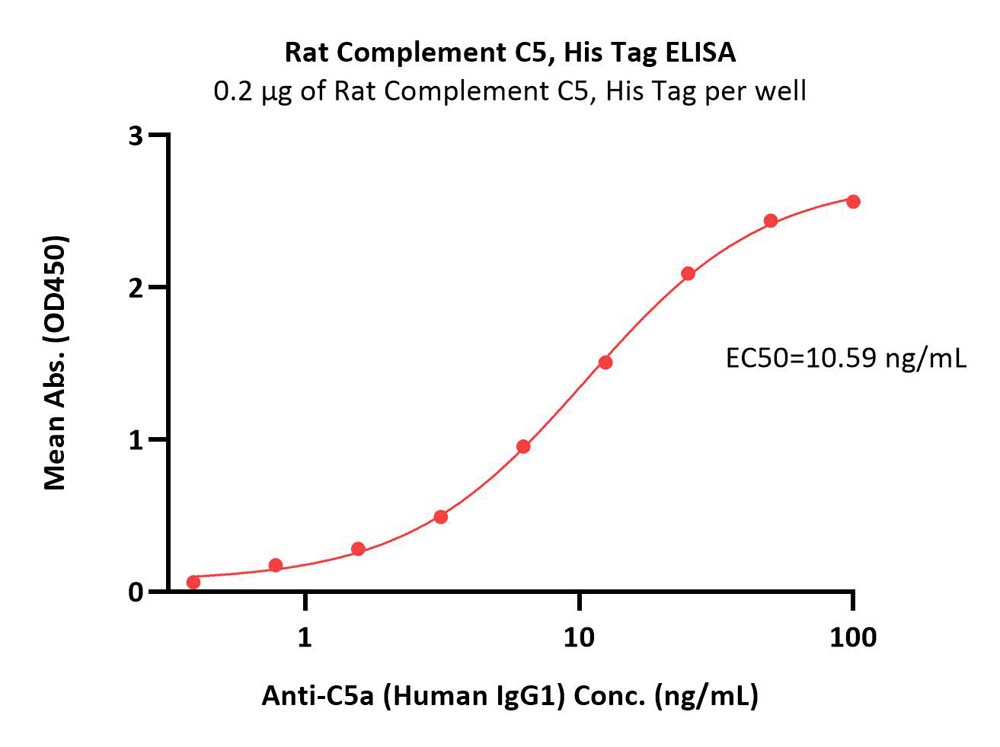
|
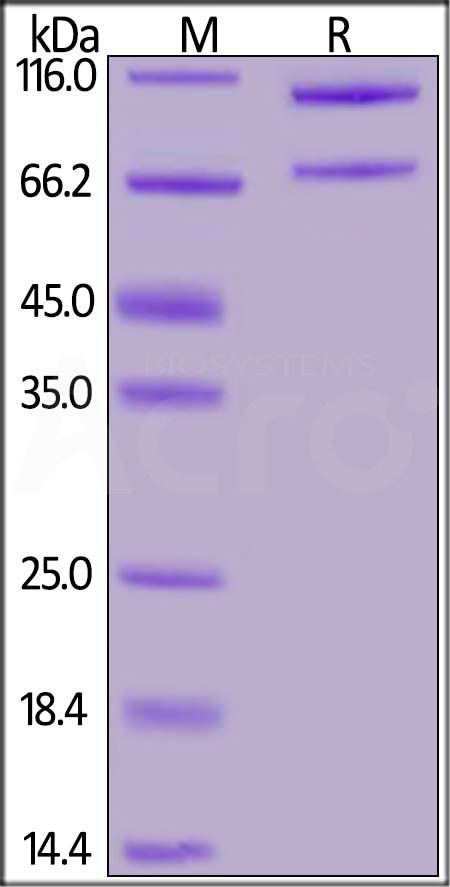
| CO5-M52H4 | Mouse | Mouse Complement C5 Protein, His Tag |  |
|
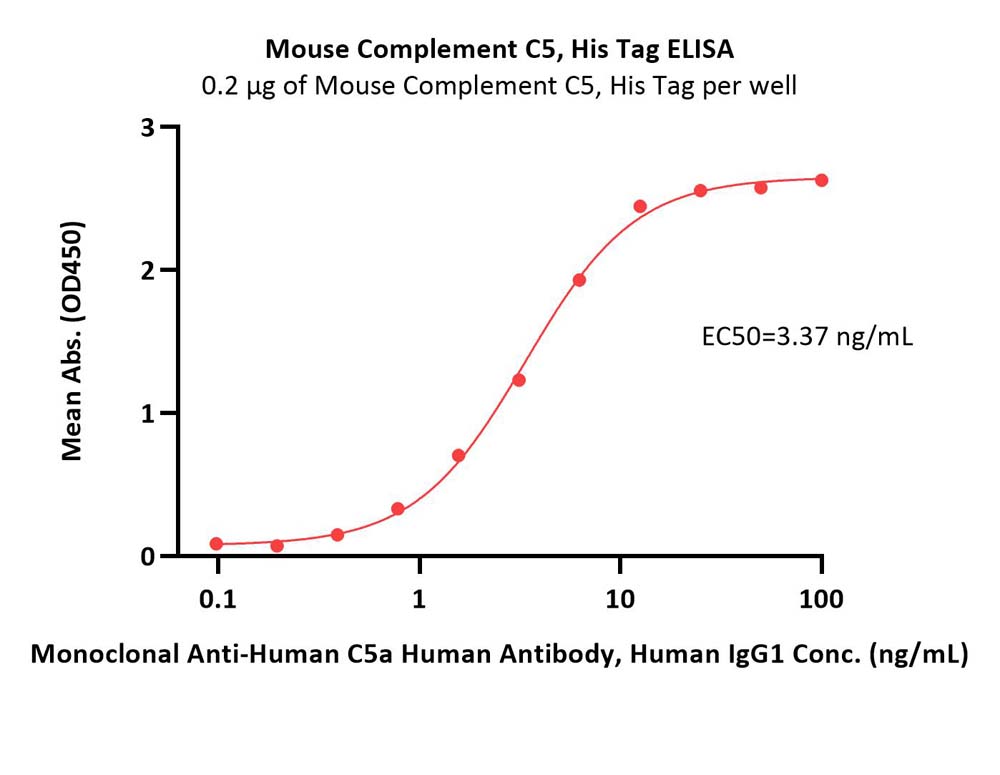
|
| CO5-H52Ha | Human | Human Complement C5 Protein, His Tag |  |

|
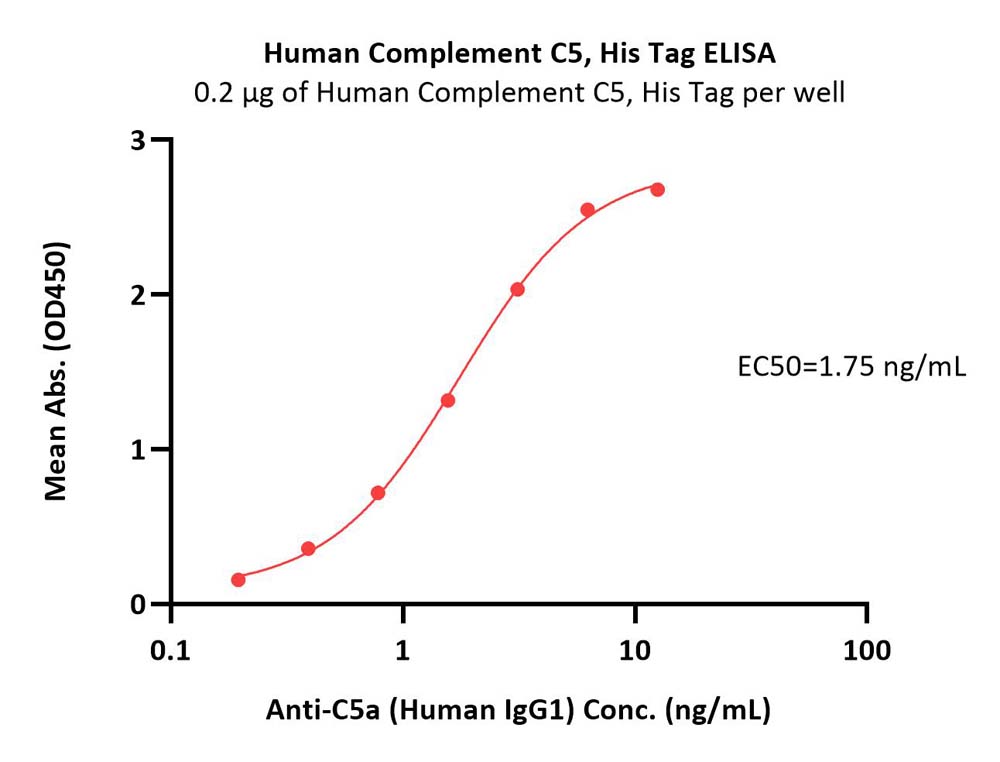
|

Immobilized Biotinylated Human Complement C5, His,Avitag (Cat. No. CO5-H82E9) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate can bind Monoclonal Anti-Human C5a Human Antibody, Human IgG1 with a linear range of 0.1-3 ng/mL (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Eculizumab | 5G1-1; HAL-1; LEX-98; h-5G1.1 | Approved | Alexion Pharmaceuticals Inc | Soliris | Mainland China | Atypical Hemolytic Uremic Syndrome; Hemoglobinuria, Paroxysmal | Alexion Europe Sas | 2007-03-16 | Neuromyelitis Optica; Macular Degeneration; Diabetes Mellitus; Kidney Failure, Chronic; Thrombocytopenia; Guillain-Barre Syndrome; Urea Cycle Disorders, Inborn; Asthma; Nasopharyngeal Carcinoma; Glomerulonephritis, Membranoproliferative; Pre-Eclampsia; Anemia, Hemolytic, Autoimmune; Myasthenia Gravis; Infant, Newborn, Diseases; Antiphospholipid Syndrome; Delayed Graft Function; Atypical Hemolytic Uremic Syndrome; Rejection of organ transplantation; Vagus Nerve Diseases; Hemoglobinuria, Paroxysmal; End Stage Liver Disease; Rejection of renal transplantation; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; HELLP Syndrome | Details |
| Ravulizumab | ALXN-1810; ALXN-1210 | Approved | Alexion Pharmaceuticals Inc, Xencor Inc | Ultomiris | EU | Myasthenia Gravis | Alexion Europe Sas | 2018-12-21 | Myasthenia Gravis; Dermatomyositis; Glomerulonephritis, IGA; Hemoglobinuria, Paroxysmal; Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Thrombotic Microangiopathies; Lupus Nephritis; Atypical Hemolytic Uremic Syndrome; Acute Kidney Injury; Neuromyelitis Optica; Pneumonia, Viral; Amyotrophic Lateral Sclerosis; Acute Lung Injury | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ALXN-5500 | ALXN-5500 | Phase 1 Clinical | Alexion Pharmaceuticals Inc | Hemoglobinuria, Paroxysmal | Details |
| Eculizumab biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Hemoglobinuria, Paroxysmal | Details | |
| Eculizumab biosimilar (IBC GENERIUM) | Phase 3 Clinical | Ibc Generium | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome | Details | |
| Vilobelimab | CaCP-29; IFX-1 | Phase 3 Clinical | Inflarx Nv | Shock, Septic; Granulomatosis with Polyangiitis; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Drug-Related Side Effects and Adverse Reactions; Microscopic Polyangiitis; Pyoderma Gangrenosum; Sepsis; Coronavirus Disease 2019 (COVID-19); Lung Diseases, Interstitial; Hidradenitis Suppurativa; Systemic Inflammatory Response Syndrome | Details |
| Eculizumab biosimilar (Amgen) | ABP-959 | Phase 3 Clinical | Amgen Inc | Hemoglobinuria, Paroxysmal | Details |
| IM-101 | IM-101 | Phase 1 Clinical | ImmunAbs Inc | Autoimmune Diseases | Details |
| Zilucoplan | RA-101495-SC; RA-101495 | Phase 3 Clinical | Ucb Sa, Ra Pharma | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Coronavirus Disease 2019 (COVID-19); Muscular Diseases | Details |
| Nomacopan | EV-576; rVA576; rEV-576 | Phase 3 Clinical | Evolutec | Hemoglobinuria, Paroxysmal; Thrombotic Microangiopathies; Pemphigoid, Bullous; Keratoconjunctivitis | Details |
| STSA-1002 | STSA1002; STSA-1002 | Phase 1 Clinical | Staidson(Beijing) Biopharmaceuticals Co Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Eculizumab biosimilar (Biocad) | BCD-148 | Phase 3 Clinical | Biocad | Hemoglobinuria, Paroxysmal | Details |
| Avacincaptad pegol | ARC-187; ARC-1905 | Phase 3 Clinical | Archemix Corp | Geographic Atrophy; Stargardt Disease; Macular Degeneration | Details |
| Gefurulimab | TPP-2511; ALXN-1720; CON-9978 | Phase 3 Clinical | Alexion Pharmaceuticals Inc | Myasthenia Gravis; Proteinuria | Details |
| P-014 | P014; KP-104 | Phase 2 Clinical | Kira Pharmaceuticals LLC | Hemoglobinuria, Paroxysmal; Lupus Erythematosus, Systemic; Glomerulonephritis | Details |
| Pozelimab | REGN-3918 | Phase 3 Clinical | Regeneron Pharmaceuticals Inc | Myasthenia Gravis; Hemoglobinuria, Paroxysmal; Protein-Losing Enteropathies | Details |
| Cemdisiran | ALN-62643; ALN-CC5; AD-62643 | Phase 3 Clinical | Alnylam Pharmaceuticals Inc | Myasthenia Gravis; Glomerulonephritis, IGA; Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome | Details |
| eculizumab biosimilar(Isu Abxis) | ISU-305 | Phase 1 Clinical | Isu Abxis Co Ltd | Hemoglobinuria, Paroxysmal; Atypical Hemolytic Uremic Syndrome | Details |
| Eculizumab biosimilar (Samsung Bioepis) | SB-12 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Hemoglobinuria, Paroxysmal | Details |
| CAN-106 | CAN-106 | Phase 2 Clinical | Beihai Kangcheng (Beijing) Pharmaceutical Technology Co Ltd | Hemoglobinuria, Paroxysmal; Genetic Diseases, Inborn | Details |
| BDB-001 | BDB-001; BDB001; BDB 001; BDB-1 | Phase 3 Clinical | Staidson(Beijing) Biopharmaceuticals Co Ltd | Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Solid tumours; Coronavirus Disease 2019 (COVID-19); Hidradenitis Suppurativa | Details |
This web search service is supported by Google Inc.
